
Diffusion welding (DFW) is a solid-state welding technique that produces a strong bond by facilitating diffusion and coalescence under controlled conditions using heat and pressure. Because it may prevent common metallurgical difficulties observed in conventional welding procedures, this specialized technology is vital in the field of metallurgy. It keeps joint corrosion resistance intact and makes it possible to fabricate components with exact dimensions, especially in titanium and zirconium. DFW is great for some high-performance applications because it can produce hefty sections with consistent qualities throughout, such as titanium laminates. For DFW joints to be successful, components need to be carefully designed and machined.
Diffusion Welding Process
In the solid-state process of diffusion welding, surfaces that have been properly prepped are brought together under precise pressure, temperature, and time constraints. Even surface contact is ensured by the applied pressure, which prevents macroscopic deformation. In order to prevent severe plastic deformation at the surfaces, the temperature utilized is typically 50% of the melting point of the metals. A filler metal, which can be plated or utilized as an insert, is frequently used. This filler metal permits welding in a less costly environment or reduces the necessary temperature, pressure, or welding time.
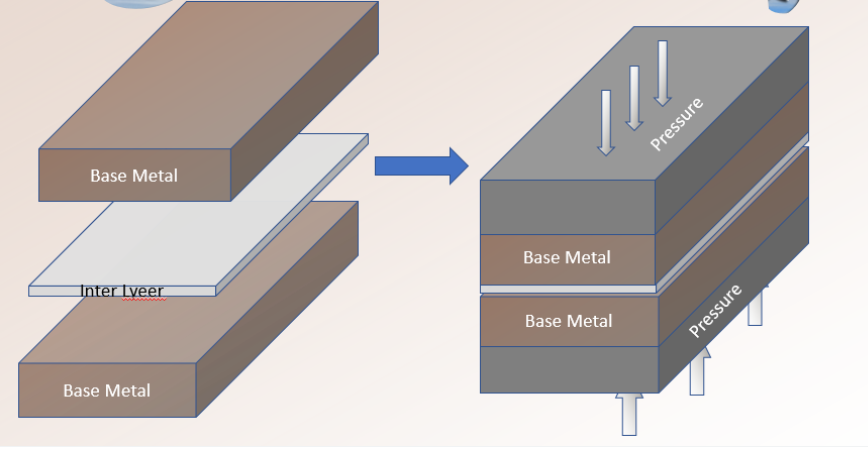
Pressure can be provided via dead weight loading, presses, differential gas pressure, or differential thermal expansion of components or tooling. Heating methods for diffusion welding include furnaces, retorts, and resistance approaches. A special set of tools for welding assemblies with intersecting flat surfaces are high-pressure autoclaves and differential gas pressure methods. However, for welding parallel planar surfaces perpendicular to the direction of load, uniaxial pressure methods are appropriate. Specialized equipment is needed for these highly mechanized processes. It is advantageous to use canning or encapsulation of parts for methods other than differential pressure approaches.
Steps in the Diffusion Welding Process
- Align the mating surfaces of the plates to ensure they are on the same plane, a critical requirement for diffusion.
- Machine, polish, and clean the surfaces thoroughly to remove any chemical impurities that could impede diffusion.
- Stack the plates together using clamps to hold them in place.
- Apply high pressure and heat to the assembly to initiate the diffusion process.
- Maintain these conditions for an extended period to allow proper diffusion to take place.
- Initially, local deformation may occur at the interface due to creep and yield processes.
- As diffusion progresses, the interface transforms and the surfaces blend together, forming a strong bond.
- Eventually, the interface line disappears, resulting in a joint with the same properties and strength as the base material.
Diffusion Principles and Mechanisms
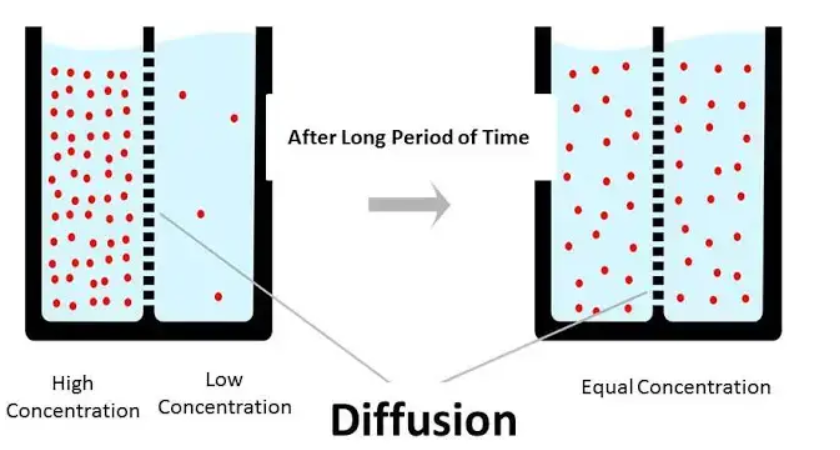
Diffusion involves the movement and redistribution of atoms, which occurs at rates dependent on the speed of migrating atoms.
Diffusion in metal systems is often classified into three processes: volume diffusion, grain boundary diffusion, and surface diffusion, according to the route that the diffusing atoms travel. Different diffusivity constants apply to each of these processes; surface and grain boundary diffusion happen more quickly than volume diffusion.
Diffusion by volume: This takes place in the majority of the substance. Due to the requirement to get beyond the energy barriers posed by the closely spaced atoms in the lattice, atom migration through the crystal lattice is often a slower process.
Grain Boundary Diffusion: This occurs at the interfaces between distinct crystals or grains in a polycrystalline material or along the grain borders. Compared to the volume, there is less order in the atomic arrangement at these boundaries, which facilitates faster diffusion.
Surface Diffusion: This happens on the material's surfaces. Surface diffusion occurs more quickly because atoms there are less firmly bonded than those within the bulk.
Diffusion according to Fick's First Law
According to Fick's First Law, the underlying formula controlling diffusion in metals is as follows:
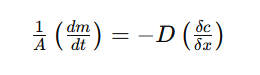
Where:
- dm/dt is the rate of flow of metal across a plane perpendicular to the diffusion direction (g/s),
- D is the diffusion coefficient (cm²/s), which varies with the metallic system, temperature, concentration, and crystal structure,
- A is the area of the plane across which diffusion occurs (cm²),
- &x/&x is the concentration gradient at the plane in question (g/cm³).
The negative sign indicates that diffusion occurs from regions of higher concentration to lower concentration.
Diffusion Coefficient and Influencing Factors
The following factors affect the diffusion coefficient D, which is not constant:
Temperature: The diffusion rate increases with increasing temperature. As a general rule, the diffusion constant doubles with a temperature increase of 11°C (20°F).
Concentration: The diffusion constant can be greatly impacted by variations in concentration. For example, at 930°C (1700°F), the diffusion constant of carbon in iron triples with an increase in carbon concentration from 0 to 1.4%.
Crystal Structure: varied crystal forms have varied diffusion rates. For instance, iron diffuses 100 times more quickly in ferrite than it does in austenite.
Crystal Directionality and Distortion: The orientation of the crystal as well as any distortions brought on by plastic deformation have an impact on the diffusion rates.
Mechanisms of Diffusion
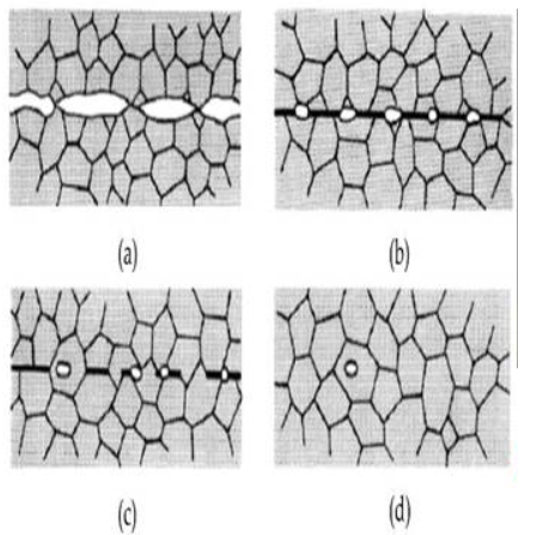
The two main ways that atoms disperse in metals are through the vacancy and interstitial mechanisms, though there are other mechanisms as well.
The movement of smaller atoms through the voids, or interstices, in the crystal lattice is known as the interstitial mechanism. These atoms are able to migrate from one interstice to another without causing major disturbances to the matrix atoms because they have smaller atomic radii than the matrix atoms.
Vacancy Mechanism: This mechanism pertains to larger atoms that are unable to fit into the interstitial spaces, such as matrix or substitutional atoms. By hopping into unoccupied lattice spaces, these atoms migrate. The fewer available vacancy sites cause the rate to be slower, even though the energy needed for these movements is comparable to that of interstitial diffusion.
Variables Affecting Diffusion Welding
One important factor in diffusion welding is time. The temperature has a major impact on how long diffusion takes. Longer durations lose their effectiveness over time. The required period needs to be ascertained empirically, as it cannot be predicted in advance. After welding is finished, more time doesn't improve the properties of the bond.
Pressure has a direct effect on diffusion welding results, particularly in the early phases. It is associated with the yield point of the constituent components, albeit figuring out a precise value in theory is difficult. For best outcomes, pressure must be exerted enough even though local deformation at the bonding spot is a natural part of the process. In order to successfully form strong bonds, it is crucial to balance heat and pressure because high compression is correlated with high equipment costs.
In diffusion welding, temperature is the most important variable. To prevent material alterations and provide a solid, stable binding, the ideal temperature must be chosen. For the welding process to be successful, proper temperature maintenance is necessary.
Equipment and Suitable Materials Used in Diffusion Welding
Specialized tools are needed for diffusion welding, including as specially designed fixtures, heat sources, presses, and autoclaves. To establish the ideal atmosphere, these tools are frequently combined with ceramic components. This technique is perfect for joining materials like titanium, aluminum, and nickel alloys, which are challenging to join using traditional techniques. Although there are less expensive ways to weld steel, diffusion welding can be a cost-effective approach for welding big, flat surfaces of low-carbon steel without the need for filler metal when the proper circumstances are fulfilled.
Advantages of Diffusion Welding
Diffusion welding has various advantages.
The resulting joint's chemical and physical characteristics are comparable to those of the parent metal. It guarantees a spotless weld that is devoid of porosity and fractures. This process is perfect for precision components because it offers high dimensional accuracy. Unlike arc welding, it may combine materials that are similar or dissimilar without the requirement for filler material. Welding is a low-cost technology that can be used to join complex shapes and materials effectively. It circumvents the difficulties associated with fusion welding and is easy to use. Diffusion welding is also very efficient and automated, requiring little expert work because it can attach several pieces in a single setup.
Disadvantages and Limitations of Diffusion Welding
Diffusion welding provides several advantages, but it also has some disadvantages. The equipment is costly, particularly for big weldments, and necessitates a specific setup with exacting cleaning and surface preparation. It is not ideal for high production rates because it requires a protected atmosphere or vacuum and takes time. Despite modest operational costs, the initial setup is expensive. Workpiece preparation is important but can be difficult. The machinery restricts the size of the welds, and there aren't many options for inspection. Due to its great reliance on exact welding parameters (temperature, pressure, surface finish, and materials utilized), the process is not appropriate for mass manufacturing. Special consideration must also be given to the various thermal expansions of materials.
Applications of Diffusion Welding
Diffusion welding (DFW) is widely used in industries like aerospace and nuclear for joining high-strength and refractory metals. A notable example of the widespread use of DFW in the aerospace industry is the engine mount on the space shuttle, which is made up of 28 titanium pieces that are diffusion welded together to manage three million pounds of thrust. DFW is also used in the fabrication of tubes with a maximum size of 203 mm by 255 cm by 457 cm. Utilizing DFW for the first time in a rotating engine component, the gas turbine industry is able to create Ti-6%Al-4%V components for advanced high-thrust engines. For these difficult applications, DFW makes possible the strong, high-performing structures that are required.


