Have you ever pondered how aeronautical components endure harsh environments or how electronic devices stay durable? Electroplating is a method that frequently holds the answer. But why is electroplating so important, particularly in modern industries where performance and accuracy are necessary?
Electroplating, a method that has become the foundation of modern manufacturing, is one such essential procedure. In sensitive applications, electroplating improves material characteristics, guarantees longevity, and increases performance in semiconductor devices and aeronautical components. But what makes this procedure so crucial for advanced technologies?
Electroplating, which began modestly as a decorative technology in the 19th century., has evolved into an essential industrial process Thanks to developments in materials science and engineering, it can now satisfy the needs of high-tech industries
Electroplating is essential for items that must function in harsh or delicate conditions because it improves performance and prolongs the life of vital components. For example, nickel plating gives aircraft components endurance, while gold electroplating ensures dependable conductivity in smartphone connectors. These uses demonstrate the importance of electroplating in facilitating high-tech developments.
Electroplating improves the mechanical, chemical, and physical characteristics of materials, from the complicated circuitry in semiconductors to the strong surfaces of aircraft components. It provides solutions to significant problems like electrical conductivity, corrosion prevention, and wear resistance. However, why is this procedure essential to industries aiming for excellence?
So, are you ready for a rollercoaster ride? We are going to explore electroplating in detail. We will learn about its role in high-tech industries and why it is still a vital component of manufacturing innovation and quality as we examine its science and applications.
1. Historical evaluation
Ancient artistry and contemporary scientific discoveries both contribute to the long history of electroplating, the technique of using electrical current to deposit a thin layer of metal onto a substrate. Modern electroplating techniques first appeared in the early 19th century, however primitive techniques for gilding and covering metal can be traced back to ancient civilizations like Mesopotamia and Egypt.
Although his work was mainly disregarded at the time, Luigi Brugnatelli used Alessandro Volta's voltaic pile to demonstrate gold plating for the first time in 1805. John Wright's discovery of potassium cyanide's efficacy as an electrolyte for plating in the 1830s led to significant breakthroughs, and in 1840, George and Henry Elkington patented a refined, commercially successful electroplating method.
Developments in electrical engineering and chemistry enhanced electroplating methods in the late 19th and early 20th centuries The method's accuracy and efficiency improved through the creation of more stable electrolytic solutions and improved control over current density. During this time, electroplated nickel and chromium coatings gained popularity, which was especially important for the home and automotive industries.
Modern Developments (Late 20th Century to Present)
In the late 20th and early 21st centuries, efforts were made to make electroplating more environmentally sustainable. Less hazardous substitutes were used in place of conventional electrolytes, such as those containing cyanide. To lessen the environmental impact of electroplating procedures, waste management systems, and recycling technologies were established.
Electroplating is still developing today due to developments in material science and nanotechnology. Contemporary uses consist of:
Semiconductor Manufacturing: Complex circuits in microchips are made using copper electroplating.
Medical Devices: Electroplated coatings in medical devices improve their longevity and biocompatibility.
Automotive and aerospace: Performance and safety are enhanced by corrosion-resistant, lightweight coatings.
2. Basics of Electroplating
Electroplating is carried out using the principles of electrochemistry, electroplating is a regulated technique that deposits a thin, homogeneous layer of metal onto a substrate. Here's a thorough explanation of how it operates:
Preparation of the Substrate
- Cleaning: To eliminate impurities like oils, grime, or oxidation that can impede the coating process, the substrate (base material) is carefully cleaned. Usually, degreasers, ultrasonic cleaning, or chemical etching are used for this.
- Surface Activation: To improve adhesion during plating, the substrate may be treated with chemical solutions to activate its surface following cleaning.
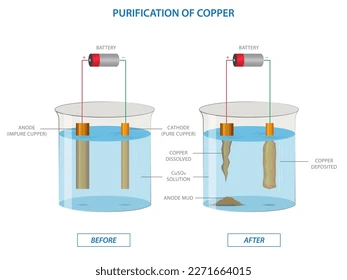
Electrolyte Solution
- The electrolyte, commonly known as the plating bath, is made up of the substance to be plated's dissolved metal ions. For instance, there are gold ions in a gold-plating solution.
- Achieving the required plating properties, like thickness and homogeneity, requires careful control of the electrolyte's composition, including pH levels, temperature, and additives.
Electrodes and Electric Current
- The anode (positive electrode) is usually composed of the plating material or an inert material such as graphite, whilst the substrate serves as the cathode (negative electrode).
- The electrolyte's positively charged metal ions migrate toward the cathode when an electric current is supplied, where they pick up electrons and form a solid metal layer.
The Deposition Procedure
At the microscopic level, the deposition procedure guarantees a uniform and even coating.
Depending on the application, the metal layer's thickness can vary from microns to millimeters, which is determined by the length of the plating process.
Post-Processing
After plating, the component is frequently rinsed to remove any remaining chemicals. It could also undergo further procedures, such as polishing or heat treatment, to improve the coating's strength and appearance.
Typical Electroplating Materials
Electroplating uses a variety of metals, each with special qualities suited to particular industrial uses:
1. Gold
- Characteristics: Outstanding electrical conductivity, tarnish resistance, and biocompatibility.
- Applications: Because of its dependability and resistance to corrosion, it is widely used in medical devices and electronics (such as circuit boards and connectors).
2. Silver
- Properties: Reflective finish, greatest electrical and thermal conductivity of any metal.
- Applications: Located in solar panels, electrical connectors, and ornamental objects.
3. Nickel
- Properties include hardness, resistance to wear, and the capacity to form a corrosion barrier.
- Applications: Frequently utilized for parts that need to be durable in industrial, automotive, and aerospace machinery.
4. Chromium
- Properties include a shiny, mirror-like polish, high hardness, and exceptional wear resistance.
- Applications: Often used in tools, ornamental fittings, and vehicle parts.
5. Copper
- Characteristics: Excellent thermal and electrical conductivity and robust metal-to-metal adhesion.
- Applications include plating electrical components and serving as an underlayer in multi-layer plating procedures.
6. Zinc
- Properties: Outstanding corrosion resistance, particularly when it comes to keeping steel parts from rusting.
- Applications: Mainly utilized in the construction and automotive industries for galvanization.
Why Material Selection Is Important
The selection of plating material is crucial and is influenced by various aspects, including:
- The desired physical characteristics, like conductivity and hardness.
- Situations that the component will encounter in the environment, such as exposure to dampness and high temperatures.
- Standards for aesthetics (such as reflecting coatings).
Knowing the specifics of the electroplating procedure and the materials involved helps to explain why it is essential for creating high-performance parts for a variety of high-tech businesses.
3. Steps in the Electroplating Process
A multi-step procedure called electroplating provides accurate metal deposition on a substrate. To achieve the required coating quality and endurance, each step is essential. Here's a thorough breakdown of the procedures:
1. Substrate preparation
For the plating substance to adhere strongly, the substrate must be properly prepared.
➢ Degreasing and Cleaning:
- To get rid of any oxidation, oil, grease, or dirt, the substrate is carefully cleansed.
- Alkaline cleaning baths, solvent degreasing, and ultrasonic cleaning are some methods.
- Weak adhesion and uneven plating may result from improper substrate cleaning.
➢ Methods of Surface Activation:
- To increase its receptiveness to plating, the cleansed surface is activated.
- Abrasive blasting, pickling, and acid etching are some methods that can be employed to produce a micro-textured surface that improves the metal layer's ability to adhere.
2. The Process of Electroplating
In the actual plating procedure, an electric current is used to carefully install the metal layer.
Bath Preparation:
- Metal salts and other plating-enhancing additives (such as brighteners and levelers) are dissolved to create the electrolyte solution.
- To guarantee consistent outcomes, the bath's composition, pH, and temperature are meticulously regulated.
Component Immersion:
- A metal or inert anode and the substrate (cathode) are submerged in the plating bath.
- Correct component placement is necessary to prevent shadowing or uneven deposition.
Monitoring and Control of Parameters:
- Important variables including bath temperature, voltage, current density, and plating time are constantly tracked.
- Temperature affects the rate of reaction, whereas current density controls the thickness and homogeneity of the plating layer.
- Automated systems are frequently employed to ensure exact control and uniformity.
3. Post Processing
The component goes through finishing procedures to guarantee quality and performance following the electroplating process.
Cleaning and drying:
- To get rid of any remaining electrolyte or plating bath chemicals, the plated component is rinsed in clean water.
- To guarantee that all pollutants are removed, several rinse steps can be necessary.
- Depending on the component and the application, drying techniques can include heat drying, air drying, or compressed air.
Final Quality Assurance Inspection:
- The part is examined for flaws including peeling, pinholes, or uneven coating.
- Methods such as visual examination, adhesion testing, and thickness testing guarantee that the plated layer satisfies client requirements and industry standards.
- For an accurate examination of the coating, sophisticated techniques like scanning electron microscopy (SEM) or X-ray fluorescence (XRF) may be employed.
Every stage of the electroplating process is essential to producing a functional, long-lasting, and high-quality coating that satisfies the exacting standards of high-tech sectors.
5. Types of Electroplating
There are several types of electroplating, each intended to satisfy particular functional, aesthetic, or industrial needs. An extensive summary of the many forms of electroplating is provided below:
1. Decorative Plating
Purpose: The goal of decorative plating is to improve component appearance while offering a minimal degree of corrosion protection.
Material: Commonly utilized materials include gold, silver, nickel, and chrome.
Applications: Located in timepieces, jewelry, car trims, and home fixtures.
Features: Shiny, smooth, and aesthetically pleasing finishes are prioritized for aesthetic appeal.

2. Protective Plating
Purpose: The protective plating function is to protect components from environmental elements such as wear, corrosion, and moisture.
Materials: Their anti-corrosive qualities, zinc, tin, and cadmium are commonly used.
Applications: Widely utilized for parts subjected to extreme environments in the construction, automotive, and aerospace industries.
Features: Increases the substrate's lifespan and is frequently utilized in outdoor or maritime settings.
3. Functional Plating
Purpose: Functional plating enhances particular chemical or physical characteristics, including wear resistance, hardness, or conductivity.
Material: Copper, nickel, silver, and gold were the materials used.
Applications: Crucial in precision-required aircraft components and electronics (such as circuit boards and connectors).
Features: Designed to fulfill performance standards instead of aesthetics
4. Selective Plating
Purpose: The goal of selective plating is to reduce material usage and expense by plating certain regions of a component.
Process: To isolate the areas to be plated, masks or fittings are frequently used.
Applications: Used to selectively coat surgical tools or connectors in industries such as electronics and medical equipment.
Features: Reduces waste and is cost-effective.
5. Brush Plating
Purpose: Localized plating is made possible without submerging the entire component in a bath.
Method: In brush plating it uses a portable brush soaked in a plating solution to apply metal ions.
Applications: Perfect for fixing worn-out or broken components, particularly in the automotive and aerospace industries.
Features: Convenient and portable for small-scale plating projects or on-site maintenance.
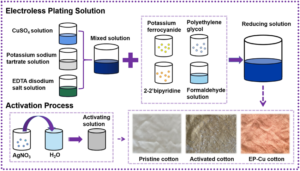
6. Electroless Plating
Purpose: The goal of electroless plating is to deposit metal layers by utilizing chemical processes rather than an electric current.
Materials: In electroless plating, nickel and copper are frequently utilized.
Applications: Widely used for uniform coatings on medical equipment and printed circuit boards in the electronics industry.
Features: Offers uniform covering for intricate forms and difficult-to-reach places.
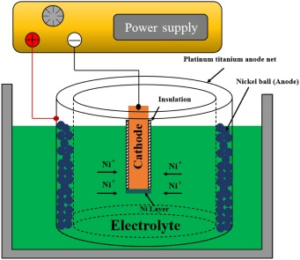
7. Hard Chrome Plating
- Purpose: The goal of hard chrome plating is to increase wear resistance and decrease friction by applying a thick, long-lasting layer of chromium.
- Applications: Found in hydraulic systems, heavy machinery, and engine parts.
- Features: Provides reduced surface roughness, remarkable toughness, and corrosion resistance.
8. Barrel Plating
- Purpose: The goal of barrel plating is to increase efficiency and cost-effectiveness by concurrently plating several tiny components.
- Procedure: The electrolyte solution and electrodes are put in a revolving barrel with the parts to guarantee even coating.
- Application: Examples of applications include the production of electrical connections, screws, and tiny fasteners.
- Features: Perfect for producing large quantities of small parts that don't need precise measurements.
6. Applications of Electroplating
The capacity of electroplating to improve the physical, chemical, and aesthetic qualities of materials makes it popular across a wide range of sectors. Here are specific examples from a variety of industries:
1. The Electronics Sector
Electroplating's capacity to enhance conductivity and inhibit corrosion makes it indispensable in the production of electronic equipment.
Applications: Connectors, switches, and circuit boards can be plated in gold or silver. Tin coating for electrical component solderability
2. Automotive Industry
Vehicle components are made more durable and aesthetically pleasing through electroplating.
Applications include chrome plating for moldings, grills, and bumpers. To stop corrosion, bolts, nuts, and other fasteners are plated with zinc.

3. The aviation sector
For components in aerospace that are subjected to harsh environments, electroplating is essential.
Uses: Nickel and chromium plating for turbine blades and jet engine components. Using cadmium plating to prevent corrosion in landing gear systems
4. Medical Sector:
Electroplating is necessary for medical devices to be precise, long-lasting, and biocompatible.
Applications include implant and surgical instrument gold plating. Medical equipment with silver plating has antimicrobial qualities.
5. The Fashion and Jewelry Sector
Fashion accessories are frequently electroplated to improve their value and look. Applications include the plating of jewelry, watches, and ornamental objects in gold or silver. For a tarnish-resistant, reflective finish, use rhodium plating.
6. Manufacturing Industry
Electroplating improves the longevity and performance of industrial tools and machines. Applications include machine parts, dies, and molds that require hard chromium plating. For industrial equipment to be resistant to corrosion, nickel plating is used.
7. Energy Sector
Electroplating facilitates power generation and renewable energy technology. Applications: To increase conductivity, solar panels can be silver-plated. Turbine blades in power plants are electroplated to increase their resistance to wear.
8. Telecommunication system
Electroplated components are essential to dependable communication systems. Applications include contacts and connectors in telecom equipment that are gold-plated. Benefits: Guarantees enduring performance and little signal loss.
9. Construction Sector
Electroplating structural elements ensure their longevity and resistance to corrosion. Applications include the plating of steel fasteners and structures with zinc. Benefits: It increases the lifespan of materials and prevents rust.
7. Advantages of Electroplating
Although electroplating has many advantages, it is necessary in many different sectors. These benefits range from cost reductions to functional and aesthetic improvements.
1. Enhanced Corrosion Resistance
Through the process of electroplating, the substrate's surface is protected from corrosive substances, chemicals, and moisture. For instance, zinc plating is frequently used to prevent steel parts from rusting in outdoor or maritime settings.
2. Improved Durability and Wear Resistance

Electroplating increases the strength and durability of components by applying a layer of durable metal. For example, hard chromium plating is frequently used to mill components and tools to reduce wear over time and endure friction.
3. Aesthetic Appeal
Components' visual appeal is increased by electroplating, which gives them a smooth, appealing surface. The bright, ornamental surfaces of jewelry, watches, and home fixtures are made from electropolishing metals like nickel, silver, and gold.
4. Electrical and Thermal Conductivity
Reliable electrical connections and enhanced conductivity are achieved by plating electronic components with metals such as copper, silver, and gold, which are excellent conductors. This is essential for circuit boards, connectors, and switches in the electronics sector.
5. Cost-Effective
Electroplating produces a high-value surface coating of precious or particular metals while enabling the use of less costly base materials (like steel or plastic). This lowers material costs without sacrificing functionality.
6. Better Adhesion and Surface Properties
A more homogeneous and useful component can be produced by electroplating small flaws or smoothing uneven surfaces. Additionally, it improves the adherence of later coats like paint or glue.
7. Chemicals and Environments Resistance
Excellent resistance to strong chemicals, high temperatures, and UV radiation is offered by plating materials like nickel or chrome. Because of this, electroplated parts can be used in harsh settings like automotive and aerospace.
8. Personalization and Adaptability
Certain characteristics, such as thickness, hardness, or color, can be achieved by customizing the electroplating process. It can be applied to a variety of materials, such as metals, polymers, and ceramics, enabling a wide range of industrial applications.
9. Fixing and Restoring
Electroplating can be used to return worn-out or damaged parts to their original size and usefulness. In the heavy industrial and aircraft industries, where part replacement can be costly, this is especially beneficial.
10. Advantages for the Environment
The procedure is more sustainable because of modern electroplating techniques that minimize waste and recycle resources. Closed-loop systems, for instance, lessen the negative effects of effluent and plating baths on the environment.
8. Challenges and Limitations
Although electroplating has many benefits, there are drawbacks as well that need to be considered to guarantee sustainability and peak performance. A thorough examination of these difficulties can be found below:

1. Environmental and Health Issues
Hazardous Chemicals: Electroplating uses hazardous chemicals such as heavy metals, cyanides, and acids, which can be harmful to both human health and the environment if improperly handled.
Waste Management: To avoid contamination, plating bath solutions and wastewater containing hazardous materials must be disposed of using strict treatment procedures.
Regulatory Compliance: Industries have to abide by stringent environmental laws, which can make operations more complicated and expensive.
2. Excessive Energy Use
High operating expenses result from electroplating techniques' frequent need for large amounts of energy to maintain the required electrical currents and solution temperatures. Reducing the energy footprint requires process optimization or sustainable energy solutions.
3. Difficulties with Surface Preparation
Strong adhesion and consistent plating depend on the substrate being well-cleaned and activated. Any preparation errors could lead to flaws like scorching, peeling, or uneven coatings.
4. Control of Thickness and Uniformity
It can be difficult to achieve uniform thickness, particularly on intricate pieces or complex geometries. Uneven deposition may result from differences in current density across the component's various regions, necessitating sophisticated methods or tooling modifications.
5. Limited Adherence to Specific Substances
Certain substrates, such as particular kinds of ceramics or polymers, can need further procedures (like surface modification) before electroplating can be used. The procedure involves chemical and heat temperatures that not all materials can tolerate.
6. The cost of valuable metals
The cost of manufacture is raised by the high cost of plating metals like platinum, silver, and gold. Thinner layers or alternatives are frequently used, however, in some situations, performance may be compromised.
7. Complexity of the Process
The multi-step process of electroplating necessitates close observation and management of factors like temperature, bath composition, and current density. Small variations may result in flaws that lower quality and increase the need for scrap or rework.
8. Durability and Adhesion issue
The coating may peel or delaminate under stress if the plating and substrate do not adhere well. Over time, repeated exposure to hostile conditions or mechanical wear may cause the coating to deteriorate.
9. Equipment and Upkeep Expenses
Installing and maintaining the specialized equipment needed for electroplating setups, including tanks, electrodes, and monitoring systems, can be expensive. To guarantee consistent performance and avoid contamination of the plating bath, routine maintenance is crucial.
10. Electroless plating's environmental limitations
Although electroless plating is a viable substitute for conventional electroplating, it has drawbacks of its own, including slower deposition rates and trouble producing extremely thick coatings.
9. Innovations in Electroplating
The demand for more accurate, sustainable, and efficient methods is propelling the electroplating industry's rapid evolution. While resolving long-standing issues, electroplating innovations are expanding their use in high-tech sectors. The following are some significant developments:
1. Non-Toxic Electroplating Alternatives:
- Environmentally Friendly: To lower the risks to the environment and human health, plating baths devoid of lead and cyanide have been developed.
- Green Plating Technologies: Using environmentally friendly chemicals and biodegradable additions in plating solutions is known as "green plating technologies."
- Closed-Loop technologies: Cutting-edge technologies that recuperate metal waste and recycle water, reducing their negative effects on the environment.
2. Nano Electroplating
- Nanotechnology Integration: The process of producing ultra-thin, high-performance coatings by using nanoparticles in plating solutions.
- Applications: Used to create strong, lightweight parts for electronics, medical equipment, and aircraft.
- Benefits: Enhanced electrical conductivity, corrosion resistance, and hardness
3. Integration of Additive Manufacturing and Electroplating
- Hybrid Techniques: Improving the surface characteristics of printed components by combining electroplating and 3D printing.
- Applications: Widely utilized to make strong yet lightweight parts in the automotive and aerospace industries.
- Features:Offers design freedom while preserving robustness and longevity.
4. Automation of Smart Electroplating Systems:
- Automation: Using robotics and automated systems to handle and plate components precisely.
- IoT Integration:Using linked devices to monitor plating parameters (temperature, current, and bath chemistry) in real-time.
- AI and machine learning: Predictive algorithms in AI and machine learning optimize the process, lowering errors and increasing productivity.
5. Innovative Coatings for Developing Sectors
- Hydrophobic and Superhydrophobic Coatings: Water-repelling electroplated layers that are perfect for outdoor and electrical applications are known as hydrophobic and superhydrophobic coatings.
- Anti-Microbial Coatings: Copper and silver plating are examples of anti-microbial coatings, which are used in the food and medical industries in particular to stop the growth of bacteria.
- Conductive Coatings: Develop ultra-thin, highly conductive coatings for wearable technology and flexible electronics.
6. New Developments in Plating Machinery
- Energy-Efficient Systems: Cutting-edge heating and rectifier systems to save energy.
- Customized Tanks and Fixtures: Modular configurations that can hold parts of different sizes and forms.
- Precision Tools: Improved tools, like laser-guided brushes, enable localized and selective plating.
7. Superior Quality Assurance Methods
- Real-time monitoring involves the use of sensors and spectroscopic methods to continuously analyze coatings and plating baths.
- Non-Destructive Testing: Techniques to assess coating integrity and thickness, such as ultrasonic testing and X-ray fluorescence (XRF).
8. Technology for High-Speed Electroplating:
- Technology: Methods that drastically cut down on plating time without sacrificing quality.
- Applications: Crucial for large-scale manufacturing in sectors such as consumer electronics and automobiles.
- Benefits: maintains cost-effectiveness while increasing efficiency
Conclusion
In modern production, electroplating is an essential procedure that unites durability, utility, and beauty. It is crucial in high-tech sectors including aerospace, electronics, automotive, and healthcare, and it may improve conductivity and corrosion resistance in addition to offering aesthetically pleasing surfaces.
But the difficulties with electroplating also change as industries do. Innovative solutions have been developed in response to environmental concerns, process complexity, and growing material costs. Technological developments including high-speed methods, smart systems, nano-electroplating, and ecologically friendly procedures are making electroplating a more effective and sustainable process.
The wide range of electroplating and its incorporation with new technologies are key to its development. In the ever-evolving world of high-tech industries, this tried-and-true method will continue to offer innovative solutions and maintain its relevance by accepting advancements and overcoming limits.