Even the tiniest leak in packaging can lead to massive issues, including financial loss, safety issues, or a loss of business reputation. But don’t worry! Vacuum decay testing offers a solution. Yes! It is the ultimate answer to confirm that a product’s seal is not compromised while preserving its integrity.
In this article, we will discuss how this nondestructive test improves product safety and reliability while saving manufacturers from significant financial losses. So, stay connected!
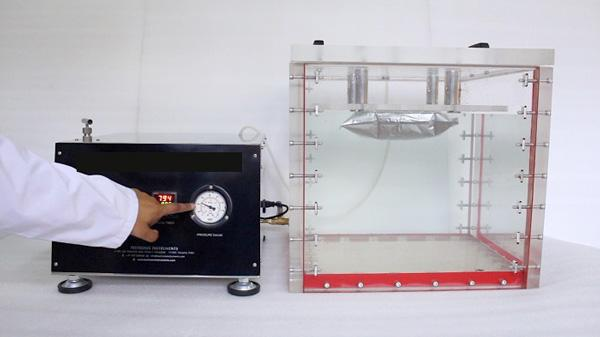
1) What is vacuum decay testing
“Vacuum decay testing is a technique performed to identify minor or major leaks within sealed packages or containers.”
The vacuum decay test is non-destructive, meaning that the product or package does not suffer damage during the testing process. So, it assures that a product’s packaging is intact and secure.
In this technique, the package needs to undergo a process where it will be placed in a sealed chamber. The air that is present inside the chamber is removed to vacuum the chamber. In case there is a leak in the package, the air from the package would escape towards the chamber, and there would be a pressure decay. Got it!
Now, this vacuum drop is very precisely measured. If the pressure gets elevated beyond a particular threshold, it implies that a leak is present. This form of testing helps ensure that a given product is not spoiled or damaged by contaminants before the customer receives it.
Moreover, all this testing does not use many resources, like time or instruments, since it is dependable, quick, and precise. The great thing is that it can even pick up very small leaks. In addition, it doesn’t damage the product, making it a useful part of quality checks.
2) Key Components of the Testing System
There are several components essential for vacuum decay testing. Each component is crucial in ensuring the accuracy of leak detection. Important components include:

i) Vacuum Chamber
This component holds the product during testing. There are many types of chambers available to accommodate various types of packages. But keep in mind the chamber must be airtight in order to maintain vacuum stability. Moreover, several chambers are designed to automate vertical testing in high-speed testing lines.
ii) Vacuum Pump
The basic function of a vacuum pump is to create a vacuum in the chamber. The decay rate for the tested vacuum relies heavily on the quality of the vacuuming pump and motor. Keep in mind that there should be no fluctuation in the vacuum level after the testing zone is attained.
iii) Pressure Sensors
These compressors have valves that fix the pressure flowing within the chamber. They must possess the potential to change by a very small value. Sensors with high resolutions increase the sensitivity of the tests. Well, accurate sensors ensure various significant enhancements in the test.
iv) Control System
This consists of the software or panel employed to control the test limits and observe the results. It handles the timing, vacuum levels, and comparisons of results. Apart from this, this system saves data for the reports as well as quality control checks. Certain systems are convenient for easy monitoring since they provide remote access.
v) Sealing Mechanism
This ensures that the chamber is completely sealed off during the test. If the seal is not accurate, the test results can be inaccurate. Keeping an optimal seal guarantees that the product alone is being tested and not the chamber.
vi) Data Display or Output Unit
The output unit shows the results in a straightforward manner, which is typically a pass or fail. In addition, some systems provide in-depth analysis, such as detailed pressure readings and graphs to enhance record-keeping.
These parts combined will enable you to have an accurate and dependable result. A Well-designed system will have strong problem-spotting capabilities and sufficient safeguards for your package.
3) Principle of Operation
The principle of vacuum decay testing is simple:
“If a sealed container has a leak, the vacuum placed around the package will allow air to enter, thereby equalising pressure inside the package with the outside environment.”
- This is how it works step by step:
Step 1) Preparation: The product is put inside a test chamber. The chamber has a specific size and shape to accommodate the product’s package. The system seals before running a readiness check and starts the test.
Step 2) Creating a Vacuum: The air gets pulled out of the chamber to make a vacuum. This step reduces pressure within the chamber to a certain level. The package, if leak-proof, will be well sealed and maintain pressure.
Step 3) Monitoring Pressure: The system has to wait and observe the chamber's pressure within a certain period. If the package has a leak, air would move from the package into the chamber, resulting in a rise in pressure.

Step 4) Pass or Fail: The system will have to perform a comparison with set boundaries. No or little pressure drop means the package meets the requirement. Going beyond the limit set in the boundaries means the package will not meet the requirement ( vacuum collapse).
This approach is efficient and reproducible. It preserves the product regardless of whether it is flexible or rigid.
4) Applications of the Vacuum decay test
Vacuum decay testing is adopted in many fields to protect specific products. It is ideal for maintaining safety, quality, and the shelf life of a product. Here are some popular uses of the vacuum decay test:
- Pharmaceutical Packaging: This method is used to check vials, ampoules, blister packs, and even syringes. The tiniest of leaks can cause contamination or the loss of sterility. So, vacuum decay aids in securing safe drug delivery.
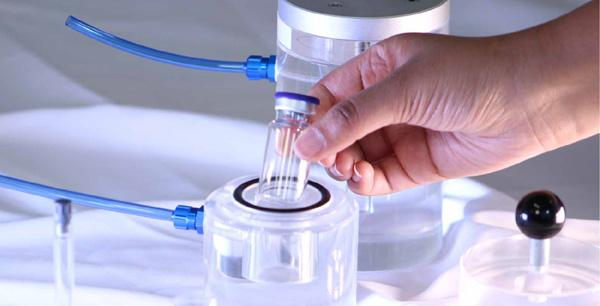
- Medical Devices: Surgical kits, catheters, and diagnostic devices are examples of such items that must be kept sterile. This test helps seal packages to avoid infection and failure.
- Food Packaging: Pouches for snacks, canned goods, and vacuum-packed meats must be free of air. The presence of air or bacteria would lead to spoilage. This technique upholds food safety.
- Beverage Containers: Both carbonated and non-carbonated drinks should be held in bottles and jars while maintaining their integrity. And, vacuum decay testing assists in checking the seal during long transport or storage.
- Cosmetics and Personal Care: Cream, lotion, serum, and other beauty products are tested to make sure they do not leak. This guarantees no alteration in quality or mess with time or even during transportation.
- Automotive and Aerospace: For safety reasons, battery packs, fuel systems, and sensors require impeccable sealing. This technique identifies minor leakages at the production line’s initial stages.
- Packaging Material Testing: Before adopting new designs or materials, manufacturers perform testing to evaluate seal retention. Vacuum decay gives fast and reliable results.
The diverse range of applications highlights the importance of vacuum decay testing. It provides a straightforward and secure way of identifying critical product and package leakage.
5) Advantages of Vacuum Decay Testing
When it comes to checking seal integrity, vacuum decay testing provides various advantages. It is simple, dependable, and widely accepted across industries. Here are the main advantages you should know:
+ Non-Destructive: There is no damage to the product and its packaging. You are able to test goods without spending any funds on them, which reduces product loss and saves money.
+ High Sensitivity: This form of testing will detect small leaks with ease. Catching problems early makes sure only tightly sealed products are released, which is advantageous for a business.
+ Accurate and Repeatable: The results are consistent and easy to trust. Accurate data is guaranteed for the vacuum decay testing because it is based on pressure change measurement.
+ Simple to Use and Automated: Modern systems are extremely user-friendly and fully automatable. Such features help save time and reduce human error. Moreover, you can test whole batches of an item in production lines.
+ Immediate Results: Testing cycles are also relatively short. Depending on the product and configuration, you can receive results in a matter of seconds to minutes. Great! This fast cycle takes your quality control efforts one step further.

+ Clean and Safe: There are no chemicals or water used, making the procedure dry and clean. You know, such cleanliness is ideal for testing more sensitive or sterile products.
+ Economic Long Term: The initial cost of the system might be higher, however, it will save money in the future through decreased waste, improved product quality, and lower risks.
+ Regulatory Approved: Vacuum decay has the endorsement of several international regulatory authorities. This includes the FDA and USP. It helps with compliance in regulated industries like pharma and medical devices.
Well, vacuum decay testing allows you to defend your brand, your product, and customers, all while using a clean and efficient technique.
6) Limitations of Vacuum Decay Testing
Despite the many advantages the vacuum decay test has, it is not without its difficulties. Understanding these will help you determine whether or not this technique is right for your product or process.
! Not Suitable for All Package Types: Very flexible or soft packages may not excel in vacuum decay testing, as they may undergo changes during the vacuum phase. So, it makes achieving accurate results challenging. Yes! It can result in inaccurate readings.
! Limit for Seal Integrity Testing: This vacuum technique is useful for finding seal leaks, but fails to provide a granulated explanation about the kind of defect and its precise location. If a detailed analysis is necessary, other testing methods will yield better results.
! Initial Set Up Cost: It is no secret that vacuum decay systems incur an expense for high-grade apparatuses. Although the long-term savings tend to offset the initial costs, the initial spending proves difficult for smaller businesses.
! Requires Proper Calibration: For accuracy to be achieved, calibration must be done competently and consistently. If calibration is not done properly, it can result in invalid results.
! Sensitive to Environmental Factors: Test results can be altered by external factors such as temperature and humidity. For the sake of precision, the apparatus has to be kept in a highly controlled environment.
! Slower for Large Volumes (if not automated): The process of running tests on individual devices is very fast, but slows down significantly when testing large batches of these devices. Automation improves this, but not all companies may have this configuration.
! Too Challenging for Porous Material: Packages made from porous materials may allow natural air flow, which can interfere with pressure measurements. So, these materials are harder to test with this method.
Alright! Vacuum decay testing is a great method for evaluating equipment, but it has its shortcomings. Well, you should consider your specific requirements relating to the level of quality your vacuum decay equipment needs, the money you are willing to spend, and so on.
7) Regulatory Standards and Validation
In the case of a vacuum decay tester, a lot of validation is necessary, and it is commonly accepted in critical sectors of the economy, especially after the invention of robots for surgery, medical devices, food packaging, and pharmaceuticals.
Requirements in vacuum decay device testing always go through strict inspection, ensuring safe and quality standards are met.

Complies With FDA and ISO Norms Standards
In the mid-1900s, decay testing of vacuum seals has proven to be a necessity in Quality Assurance Compliance in tools and devices coming from the healthcare and pharmaceutical sector. Vacuum testing meets standards set by the FDA ( Food and Drug Administration) and ISO (International Organisation for Standardisation).
These bodies define organic processes concerning the control of sealing, especially for sterile equipment. For instance, the FDA oversees control processes formulated for packaging and labelling drug products under 21 CFR part 211.
USP Section <1207>
The United States Pharmacopoeia (USP) Chapter <1207> describes the integrity testing processes for packaging sterile products. Vacuum decay is one of the approved techniques for assessing seal integrity and preservation of sterility, which is crucial in pharmaceutical and other critical care packaging. Well, this guarantees that no contamination occurs prior to consumption.
Validation of the Best Outcomes
Regulatory agencies also stress that the validation of the entire testing system must be applied. This means vacuum decay testing equipment must be proven to have reproducible, reliable outcomes within defined ranges of tested parameters. The system is only validated if it undergoes frequent calibration and routine care.
Record Retention and Documentation
Traceable documents reflecting established business processes must support compliance with defined legal obligations. All actions taken in the scope of the system, including but not limited to test results, calibration, and maintenance logs, have to be recorded for accountability. These documents need to be maintained and made available during audits or inspections.
Thus, striving to meet legislation makes vacuum decay testing a highly valued process in regulated industries concerned with product safety and compliance.
8) Conclusion
Okay! When it comes to ensuring consistency in packaging across different industries, vacuum decay testing proves to be a dependable non-destructive technique. Although there are a few disadvantages, the benefits surpass the limitations.
Vacuum decay testing upholds regulatory requirements and validates packaging quality, and plays an essential role in providing customers with reliable and safe products. No doubt, this method is crucial in any quality control procedure.





